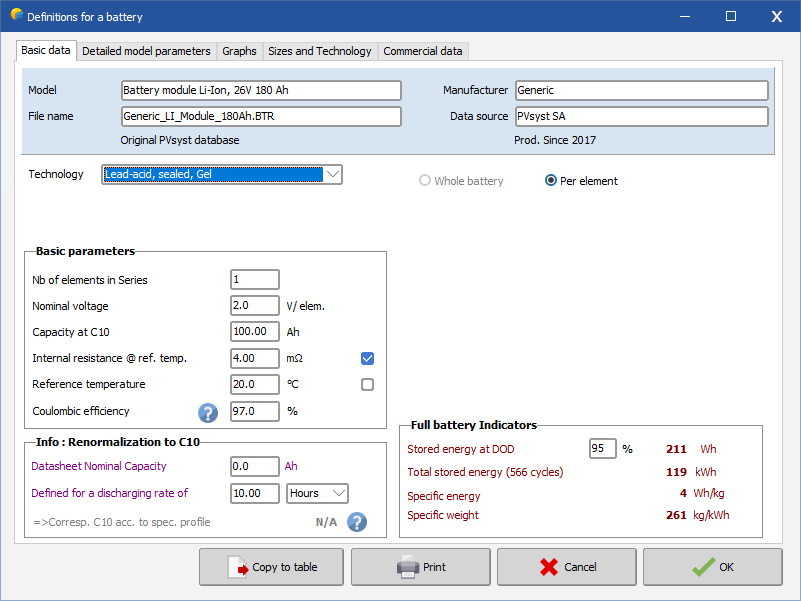
Battery identifiers:
| - | Model and Manufacturer will appear in the batteries choice lists. |
| - | Data source usually refers to the data source (most often Manufacturer, may be an independent institute or your own measurements). |
| - | File name should have the extension '.BTR' |
Technology (lead-acid)
| - | Technology specifies vented or sealed (without maintenance) batteries, and tubular / plates / vehicle starting technologies. |
| - | All Lead-acid batteries are made of 2V-elements in series (12, 6 or one element). A big capacity battery unit is 1 only element. |
Basic parameters:
The most important battery specifications present on any manufacturer data sheet.
| - | Number of elements in series, |
| - | Nominal voltage: is always 2V per element for lead-acid batteries. |
| - | Nominal capacity: the reference capacity is specified for a discharge rate in 10 hours (noted C10) and for a reference temperature of 20°C. Capacity behavior according to discharge rate and temperature will be defined in the next sheet. |
| - | Internal resistance is considered to be constant (in approximation). If not specified you can ask for a reasonable default. |
| - | Reference temperature is the reference temperature used by the model to set 2 corrections: the Open Circuit Voltage correction as a function of temperature, and the capacity correction as a function of temperature (Detailed model parameter). |
| - | Coulombic efficiency (or faradic or current efficiency), is the discharge/charge cumulative currents balance, in [Ah]. For a working range below the overload, i.e., without dissociation of the electrolyte ("gassing" phenomenon appearing at approximately 85 to 90% of full charge), it is generally approximately 97% for lead-acid batteries. |
Gassing
When the state-of-charge increases, the dissociation progressively appears, and is manifested on the one hand as an excess of voltage in relation to the normal charge curve, and on the other hand by a production of gaseous oxygen and hydrogen, which consumes a part of the charge current by electrolysis, thus disturbing the determination of efficiency. The real efficiency therefore depends on the working conditions and on the regulation. It will be quantified during simulation, and the current lost in electrolysis will give an estimation of the quantity of dissociated electrolyte.
In all of these definitions the voltage specifications may be displayed either by cell or for the whole battery.
All these parameters may be shown either for one cell, or for the whole battery.